Proposed use
TrAPs technology has significant potential via repurposing existing inhibitory aptamers for new addressable tissue repair markets including skin repair, orthobiologics/orthopaedics, angiogenesis, and neural injury. Immediate potential markets could include spinal and ankle fusion, maxillofacial reconstruction, angiogenesis, and wound repair. Moreover, synthetic synthesis strategy instead of protein engineering offers attractive manufacturing opportunities.
Problem addressed
Growth factors (GFs) have long been investigated as potentially powerful therapeutics for promoting tissue repair. Currently, a handful of GFs are in various markets for promoting the repair of tissues including bone and skin. However, these treatments generally use inefficient methods of delivery. This leads to therapeutic doses of GFs that are orders-of-magnitude higher than physiologically relevant, leading to serious side-effects and prohibitively high manufacturing costs.
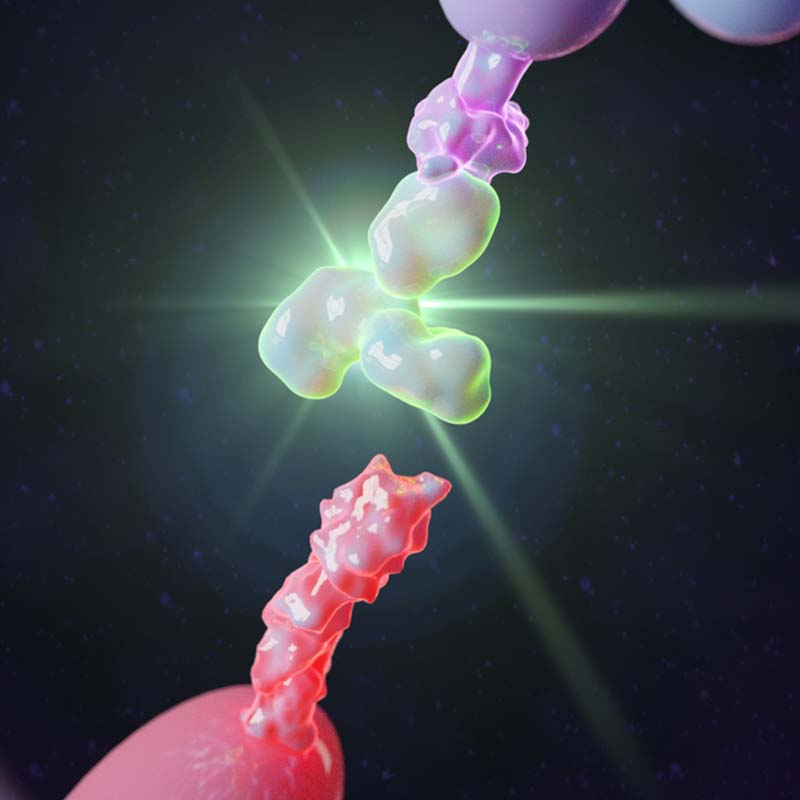
The TrAPs technology overcomes these limitations by creating an engineered platform for controllable, ‘cell-activated’ therapeutic delivery. It is the only technology available that harnesses cellular traction forces to activate therapeutics such as GF at physiologically relevant doses to locally promote tissue repair.
Technology overview
TrAPs are based on aptamers – single-stranded DNA/RNA molecules that fold into 3D secondary structures that bind and inhibit proteins with affinities and specificities rivalling antibodies, with their 3D folded structure being critical for their function. TrAPs are created by attaching a cell adhesion peptide to one end of the aptamer and the other end to a biomaterial substrate. This enables cells to directly bind the TrAPs and actively trigger the activation and release of bound proteins via traction forces that unfold the aptamer. This is a fundamentally different approach to GF delivery that has the potential to drastically reduce the GF dose necessary for therapeutic effect by increasing delivery efficacy, while also eliminating the serious side-effects associated with existing strategies.
Benefits
- Chemically synthesised using established procedures for reduced manufacturing costs
- Can potentially deliver any protein/peptide of therapeutic interest due to SELEX selection/enrichment process for aptamers
- Predicted to reduce required therapeutic GF doses by several orders of magnitude over existing technologies due to controlled, on-demand cell activation and possible synergistic integrin signalling
- Only technology to have demonstrated selective activation of GF by different cell types
- Can be incorporated into/onto any biomaterial scaffold (e.g. collagen, fibrin, cellulose, silk, PEG, PLGA, PCL, PEEK, titanium)
- Minimally-invasive
Intellectual property information
EP 17777320.7 – DRUG DELIVERY USING APTAMER CONSTRUCT
US 16/333982 – DRUG DELIVERY USING APTAMER CONSTRUCT
Link to published paper(s)
Biologically Inspired, Cell-Selective Release of Aptamer-Trapped Growth Factors by Traction Forces
Anna Stejskalová, Nuria Oliva, Frances J. England, and Benjamin D. Almquist, Advanced Materials v31, 1806380 (2019). DOI: 10.1002/adma.201806380