DTP3 is a first-in-class GADD45β/MKK7 inhibitor targeting the NF-κB pathway
Proposed use
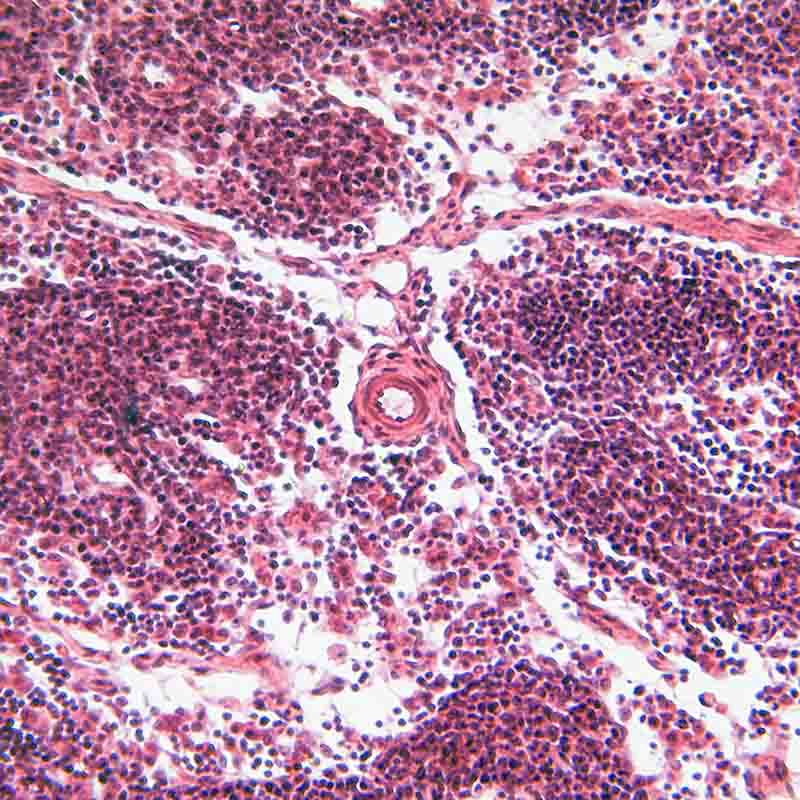
Multiple Myeloma (MM) and Diffuse Large B Cell Lymphoma (ABC-DLBCL) cells rely on aberrant NF-κB activity for survival. DTP3 disrupts this pathway, killing MM and ABC-DLBCL cells via JNK-driven apoptosis.
Additionally, GADD45β/MKK7 inhibition has been reported to be neuroprotective in two in vivo models of ischemia.
Problem addressed
There is significant clinical need for safer and more effective treatments for both MM and DLBCL. Current MM therapies rarely achieve lasting remissions, and are often too toxic for elderly patients or those with comorbidities. While more than 50% of DLBCL cases are cured with standard immuno-chemotherapy, patients who do not respond generally die from their disease.
Technology overview
The D-tripeptide DTP3 was developed through a peptide library screen to target the survival complex formed by the NF- κB regulated factor, GADD45β, and the JNK kinase MKK7.
DTP3 is highly specific and kills MM/ABC-DLBCL cells without damaging normal cells. It has a similar IC50 to the standard of care, bortezomib, but more than 100 times greater therapeutic index ex vivo.
Preclinical work has been completed, showing efficacy, specificity and tolerability, with wide safety margins.
In a proof-of-concept Phase I study, DTP3 induced markers for apoptosis in malignant CD138+ cells, but not in healthy CD20+ cells. The trial data suggests that patient stratification could occur through measurements of GADD45β expression and the extent of JNK-induced apoptosis in tumour cells.
Initially, it is envisaged that DTP3 will be introduced as salvage therapy in late-stage patients to alleviate toxicities, extend remissions and improve quality of life as a result of its enhanced safety profile and ability to bypass drug resistance.
Benefits
- DTP3 inhibits GADD45β/MKK7 with sub-nanomolar activity
- Target selective
- Kills malignant CD138+ cells ex-vivo
- Protective in murine models, with high plasma concentration and bioavailability
- Good tolerability and no reported toxicity in humans
- Biomarkers identified for patient stratification
Intellectual property information
This technology is filed in multiple territories and covers composition of matter and use (WO2011048390A2) with a separate patent family covering the patient stratification marker (WO2012118909A1).