A proposed microbiome therapeutic against CRE and VRE, comprising a synthetic microbial consortium and inhibitory metabolites.
Proposed use
The technology could form the basis of a novel microbiome therapy to inhibit CRE or VRE growth.
Problem addressed
The intestine is the primary colonisation site and reservoir for pathogens called carbapenem-resistant Enterobacteriaceae (CRE) and vancomycin-resistant Enterococcus (VRE). CRE and VRE intestinal colonisation precedes the development of other serious antibiotic-resistant infections, such as bloodstream infections or recurrent urinary tract infections. Antibiotic-mediated killing of gut microbiota members allows CRE and VRE to colonise and dominate the intestine. Antibiotics increase the concentration of nutrients available to support CRE and VRE growth, coupled with a reduction in the concentration of metabolites that can inhibit CRE and VRE growth.
Our novel approach will benefit patients by inhibiting the long-term growth of VRE and CRE, reducing the risk of developing serious infection.
Technology overview
Researchers at Imperial have identified a range of microbial metabolites that inhibit CRE and VRE growth. Figure 1 (below) shows that the growth of E.coli, K. pneumoniae and E. cloacae was inhibited through the introduction of specific microbial metabolites. The presence of these metabolites in antibiotic-treated faecal microbiota is reduced.
The proposed microbiome therapeutic consists of a synthetic microbial consortium (specific members of the gut microbiota) and these inhibitory metabolites. The introduction of inhibitory metabolites will cause an initial reduction in CRE or VRE growth. Then, the synthetic microbial consortium will colonise the intestine, outcompeting VRE and CRE for nutrients, and producing further inhibitory metabolites, resulting in long-term reduction in CRE or VRE growth.
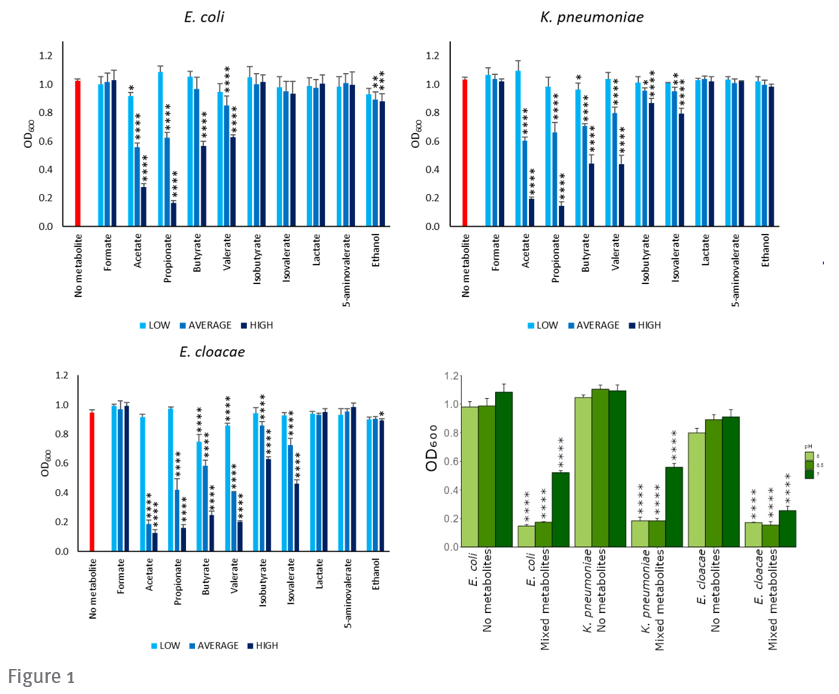
a-c) CRE growth was inhibited by individual metabolites in a dose-dependent manner. Growth of E. coli, K. pneumoniae, or E. cloacae in LB supplemented with an individual metabolite at low, average, or high concentrations (mimicking stool concentrations) or unsupplemented (no metabolite control) at pH 6.5.
d) CRE growth was inhibited by a mixture of metabolites that were decreased in antibiotic-treated faecal microbiota. E. coli, K. pneumoniae, or E. cloacae were grown in LB supplemented with a metabolite mixture (mimicking average human faecal concentrations) or unsupplemented (“no metabolite” control) at pH 6, 6.5, or 7.
Benefits
- Microbiome therapeutic approach to inhibiting long-term growth of CRE and VRE
- Removing CRE or VRE intestinal colonization reduces the risk of serious antibiotic-resistant bacterial infection
- Proposes wide spectrum of nutrients and metabolites important to the inhibition of CRE or VRE
Intellectual property information
UK Patent Application No. 2217266.2