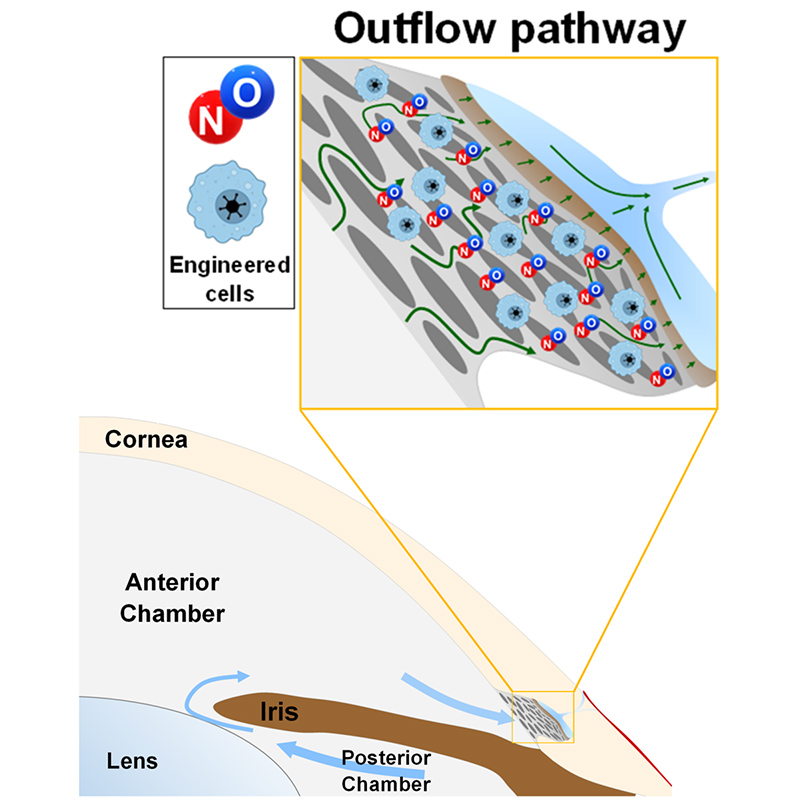
A cellular therapy to locally deliver nitric oxide (NO) to the conventional aqueous humour outflow pathway, thereby lowering eye pressure and preserving vision in glaucoma.
Proposed use
Glaucoma is a leading cause of irreversible blindness, affecting 80M people worldwide, growing to 110M by 2040. Reducing eye pressure is the only way to prevent further vision loss in glaucoma. However, current treatments often fail to achieve sufficient or sustained eye pressure reduction, mostly because they do not directly target the tissues responsible for eye pressure regulation in the conventional aqueous humour outflow pathway.
This invention proposes a new and innovative approach to enhance fluid drainage through the conventional outflow pathway to more successfully lower eye pressure and prevent vision loss in glaucoma.
Problem addressed
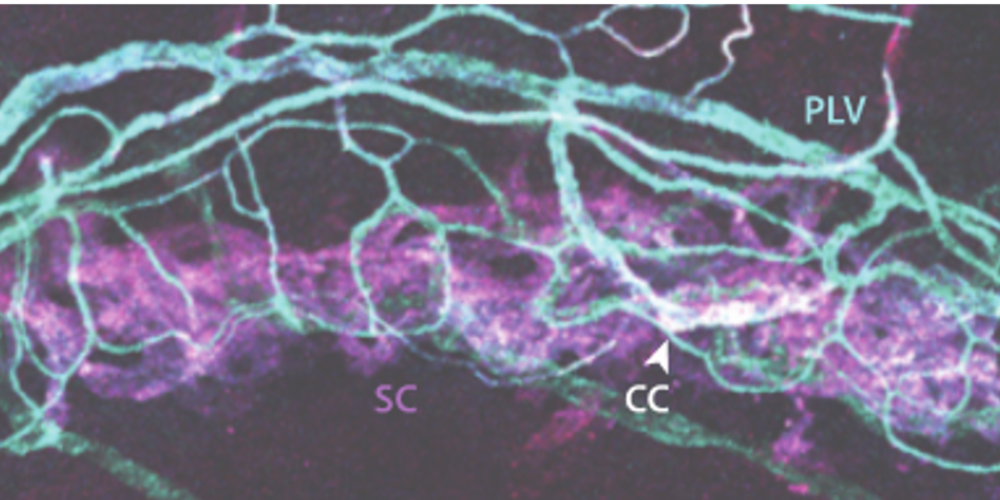
NO is known to lower eye pressure, but NO is short lived and typically cleared within minutes, complicating drug delivery. For example, NO-donating eye drops like Vyzulta (Bausch+Lomb) promise improved pressure reduction, but it remains unclear whether NO is reaching the outflow pathway. Further, all NO-donating drugs have a limited capacity for NO release, necessitating frequent dosing. To overcome these limitations, we propose a novel bioengineering approach that exploits the body’s own NO-generating enzymes to achieve sustained delivery of NO directly to the pressure-regulating tissues of the eye. An image showing the distribution of one such enzyme (eNOS) in Schlemm’s canal (SC) and perilimbal vasculature in a mouse eye is shown in the image below.
Technology overview
This invention achieves sustained cellular delivery of NO to the conventional aqueous humour outflow pathway. The technology utilises in vitro bioengineering methods to express NO-synthase, the enzyme responsible for NO production, in autologous patient-derived cells. These engineered cells are then administered into the anterior chamber, where on account of aqueous humour flow they are carried directly to the conventional outflow pathway. Pre-clinical studies support local delivery of NO to the outflow pathway as a highly efficacious method to enhance conventional outflow and lower eye pressure. We are now exploring partnerships that can help us translate this technology towards more effective clinical management of eye pressure in glaucoma.
Benefits
- Localised and sustained delivery of nitric oxide to the pressure-regulating tissues of the eye
- No additional dosing is required after first administration, which can be performed during cataract surgery
- No concerns over patient compliance