HKMTi-1-005 is a dual G9A/EZH2 histone methyltransferase which reactivates epigenetically repressed genes and inhibits tumour cell growth.
Proposed use
HKMTi-1-005 has the potential to treat any disease or disorder that is associated with G9A/EZH2 dependent activity, including: Myeloma, leukaemia, lymphoma, melanoma, small cell lung, ovarian, breast.
Problem addressed
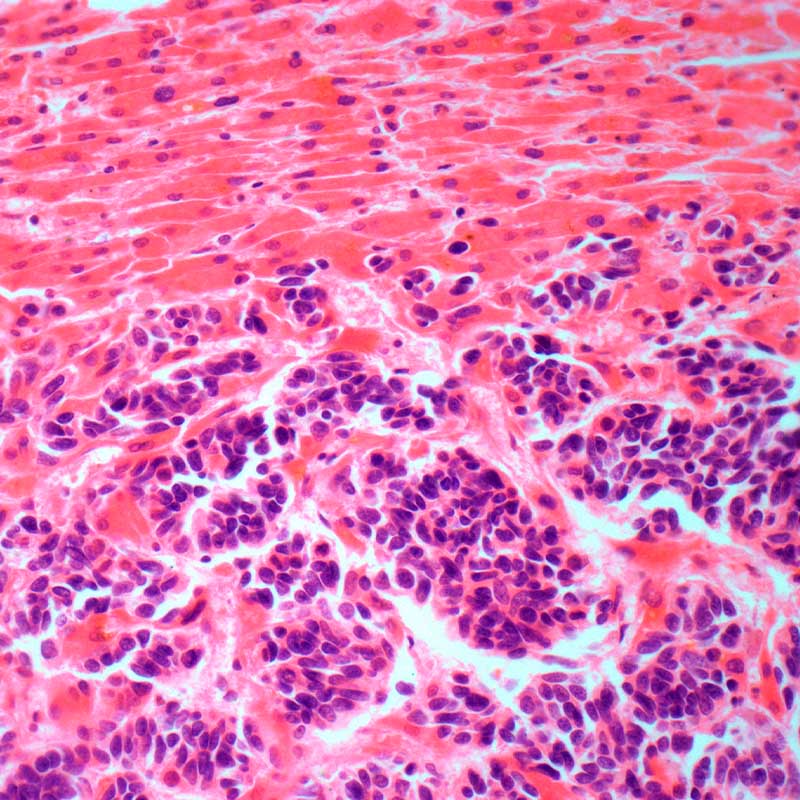
Aberrant epigenetic silencing of genes has been implicated in the aetiology of many diseases and has been observed in all cancer tumour types. Unlike other epigenetic marks which are mediated by multiple enzymes, the H3K27me3 repressive mark is maintained primarily by EZH2, making it a desirable target in the development of epigenetic therapies that reverse gene repression. EZH2 and G9A are over-expressed in many cancers, which associates with patient survival. EZH2 and G9A proteins interact with each other. Inactivation of both EZH2 and G9A has been shown to be more effective in inducing transcriptional changes and tumour growth inhibition than inhibition of either enzyme alone.
Technology overview
HKMTi-1-005, in contrast to current EZH2 inhibitors in clinical trials, is a substrate competitive, peptide binding site, inhibitor which reduces both H3K9me and H3K27me levels in the genome. HKMTi-1-005 induces expression of genes not affected by single EZH2 or G9A inhibition and copies dual gene knockdown. HKMTi-1-005 demonstrates drug-like properties, with good tolerability in mice at physiologically relevant plasma levels. HKMTi-1-005 induces growth inhibition of tumour models, which is accompanied by immunological changes in a syngeneic ovarian cancer model. HKMTi-1-005 synergises with PARP inhibitors (DNA repair targeted chemotherapy) to induce tumour cell growth inhibition. Investigators are progressing preclinical development, toxicology, oral bioavailability, in vivo xenograft studies, biomarker evaluation and patient stratification indications.
Benefits
- Highly selective inhibitor of G9A/EZH2.
- Good cell permeability.
- Well tolerated in vivo.
- Improves the survival of mice.
- Reduces tumor burden in mice.
- Stimulates anti-tumour immune responses
- Synergises with chemotherapies
- Pharmacodynamic and sensitivity biomarkers
Intellectual property information
Granted patents in UK, Germany, Spain, France, Italy and US