A technology that transforms plastic waste into green H2 and high value, sustainable chemicals at low cost.
Proposed use
This novel technology can upcycle polyethylene terephthalate (PET) plastic waste into high-quality building block terephthalic acid (TPA) while co-generating green hydrogen. The technology comprises the prototype and process to achieve full conversion of plastic waste with > 95% yield to terephthalic acid, and generates hydrogen with 50% less electricity consumption than the state-of-the-art water electrolyser technology. The high purity terephthalic acid can be reused to produce virgin PET plastic, and the clean H2 can be used as reactant or high energy carrier in chemical industry to help in decarbonising the sector.
Problem addressed
Polyethylene terephthalate (PET) in the form of beverage bottles is the most recycled plastic in the world. Yet the majority of the PET produced annually currently ends up in landfills or incineration facilities. Even for the small fractions that have been recycled, PET degrades over time through standard recycling protocols (melting and reshaping), and thus, recycled PET is normally unsuitable for producing food-grade containers due to leaching of degradation compounds.
This technology is designed to recycle PET back to its original quality, by producing high purity chemicals that can be directly reused to make virgin PET. This enables closing the PET → TPA → PET usage loop, and therefore will be extremely appealing for major food grade PET manufacturers and brand-owners. At the same time, the co-produced H2 will contribute towards the decarbonisation of the chemical sector by bringing clean and low-cost green H2 in as the primary energy source carrier and feedstock, to produce chemicals such as ammonia, methanol, etc.
Technology overview
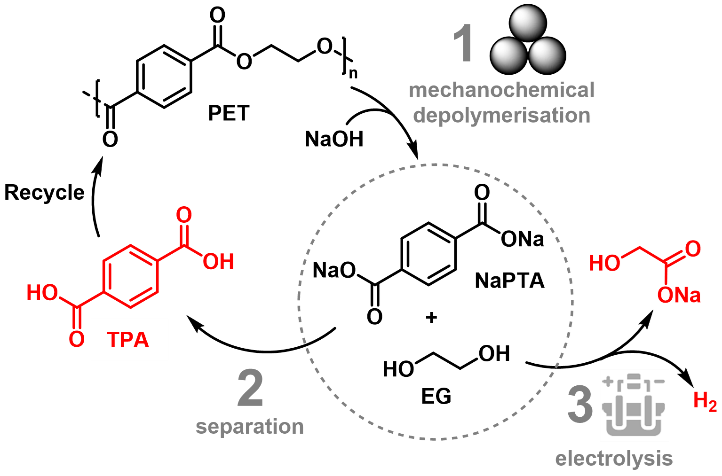
Figure 1: novel PET upcycling process
The technology introduces a new process to recycle PET into its main building blocks terephthalic acid (TPA) and ethylene glycol (EG), with subsequent electrolytic conversion of EG into clean H2. It contains three main steps: 1. Mechanochemical depolymerisation of PET; 2. Separation of TPA and 3. Electrolysis of EG, with each step being highly scalable. With just mechanical energy coupled with electrodialysis separation, we are able to produce ultra-pure TPA that can be re-polymerised to produce food packaging-grade PET. The EG electrolysis step resembles water electrolysis, with a much lower thermodynamic energy requirement. Substituting the challenging water oxidation (> 1.23 VRHE) with the partial oxidation of EG (< 1 VRHE) saves more than 50% electricity for H2 production.
Intellectual property information
PCT application number: PCT/GB2024/051009