Novel lipid nanoparticles (LNPs) for targeted delivery of RNA-based vaccines and therapeutics, with improved long-term (12 months) thermostability at ambient temperatures (as high as 40 °C), in aqueous liquid or lyophilised form.
Proposed uses
LNPs are among the most widely studied RNA delivery systems. Our novel LNP technologies can enable not only targeted RNA delivery with enhanced efficacy but also long-term thermostability at room and tropical temperatures without need for cold chains, thus solving the major challenges on development, storage and distribution of RNA-based vaccines and target therapies.
Problems addressed
Research and development into nucleic acid-based vaccines and therapies is currently among the most promising to address some of the world’s prolific diseases and illnesses, such as the COVID-19 pandemic. RNA is a key asset in pharma/biotech pipelines for vaccines and target therapies. However, nucleic acids in particular RNA have low cellular uptake, endosomal entrapment, easy degradation, and poor biodistribution, causing inadequate protein expression to provide immunity and therapeutic effects. In addition, they have a short shelf-life and require very challenging cold-chains, causing high deployment costs and complexity. Currently, it remains a major hurdle to addressing significant challenges of efficient RNA delivery and non-cold-chain storage together.
Technology overview
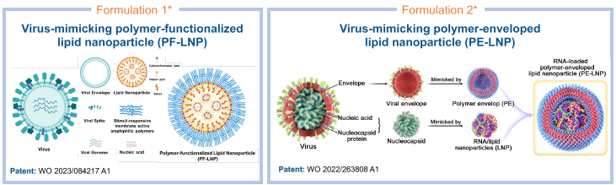
A team from Imperial College London has developed a new generation of bio-inspired LNP technologies for targeted delivery and non-cold-chain storage of RNA-based vaccines and therapeutics. Viruses might be negatively associated with illness, but they have evolved to efficiently enter cells and replicate whilst protecting their viral RNA cargo from degradation, even at tropical temperatures. The two patented virus-mimicking formulations, the polymer-functionalised lipid nanoparticle (PF-LNP) and polymer-enveloped lipid nanoparticle (PE-LNP) as shown in Figure 1, have been developed to leverage these advantageous properties. The formulations can mimic the viral spikes of a virus to enhance RNA delivery efficacy, the viral envelope to protect the RNA cargo, and the nucleocapsid to enhance delivery and stability. Both cutting-edge formulations show better delivery and protection of payloads including RNA for different applications, compared to the conventional formulations in the market.
Benefits
- Efficient targeted delivery of RNA and other payloads with enhanced efficacy.
- Long-term (12 months) thermostability at ambient temperatures (as high as 40 °C), in liquid or lyophilised form (commercial formulations: cryogenic storage at –70 °C/–20 °C).
- Simple one-pot, scalable manufacturing; biocompatible, non-toxic; FDA-approved compositions.
- The low-cost solution, and versatile applications for vaccines and targeted therapies.
Intellectual property information
Polymer-Enveloped Lipid Nanoparticle (PE-LNP), Imperial reference number 10803
- Sub-Micron Particle, Japan, application number: 2023-577253
- Sub-Micron Particle, Europe, application number: 22732305.2
- Sub-Micron Particle, US, application number: 18/570611
- Sub-Micron Particle, China, application number: 202280055882.4
Polymer-Functionalized Lipid Nanoparticle (PF-LNP), Imperial refence number 10958
- Payload Delivery System, PCT application number: PCT/GB2022/052847