A nanoparticle drug platform that can deliver small molecule drugs over a sustained period in a stable, low-toxic manner.
Proposed use
This novel nanoparticle drug delivery technology is poised to revolutionize the pharmaceutical industry, particularly in segments focused on chronic conditions requiring long-term medication. It promises to benefit patient groups with diseases like arthritis or diabetes, where controlled, sustained drug release is crucial. Other potential patient groups include those taking chronic prophylactic medications. Market analysis suggests a significant demand for such innovations, with potential expansion into other sectors seeking slow-release solutions. For example, radiotherapeutics for labelling and diagnostics in PET sensing as well as chemotherapies.
Problem addressed
The technology confronts the instability of conventional drug delivery systems, which often degrade quickly and release drugs inconsistently, causing fluctuating drug levels that can lead to toxicity. The nanoparticles remain stable, ensuring steady drug release and minimising side effects. Additionally, the technology successfully navigates the Blood-Brain Barrier, as evidenced by therapeutic changes in stroke models. The synthesis of covalently crosslinked nanoparticles via nano-polymerization is a key innovation, allowing for sustained drug release without the adverse effects of prolonged drug presence.
Technology Overview
This innovative nanoparticle platform marks a significant advancement in drug delivery. The ‘nanocapsule’ is the first of its kind, featuring a fully covalent bond structure for enhanced safety and stability. It can encapsulate diverse therapeutic agents with precision. The platform’s unique chemistry and polymerisation process yield nanoparticles that set new benchmarks in longevity and controlled drug release, preventing premature dispersal. Its robust performance in physiological conditions and its ability to facilitate a slow, regulated release make it a compelling choice for pharmaceutical applications. The nanoparticles also show potential in enhancing therapeutic effectiveness, reducing toxicity, and reviving interest in previously ineffective bioactives. Successful human cell uptake, indicated by uniform distribution in fluorescein-labelled HeLa cells, validates the initial design and functionality of the nanoparticles.
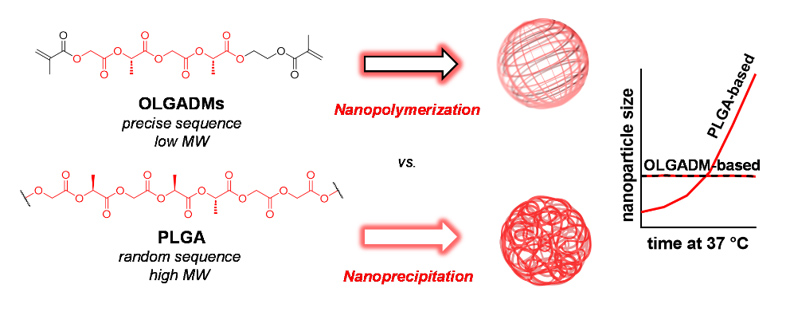
Figure 1 Oligolactoglycolic acid dimethacrylates (OLGADMs) can nanopolymerise In a controlled uniform manner vs conventional PLGA-based nanopolymers.
Benefits
- Consistent Delivery: Provides an avenue for predictable and steady drug release, enhancing treatment efficacy.
- Extended Duration: Offers a stable drug presence, far exceeding current solutions.
- Safety and Stability: The unique covalently crosslinked chemical structure ensures safety and resistance to premature breakdown and inertness to bioactive and the body.
- Innovative Chemistry: Employs cutting-edge chemical synthesis for unparalleled precision in drug delivery.
Intellectual property information
Patent application has been filed